Kovaltry demonstrated efficacy data in ABR for children with at least 2x/week prophylaxis infusions1*
- The median ABR in children ≤12 years old was 0 (Range: 0.0–12.0) for spontaneous, 0 (0.0–17.7) for traumatic and 0 (Range: 0.0–15.8) for joint bleeds1,5*†‡
ABR in children in LEOPOLD Kids (Part A)1,5*†‡
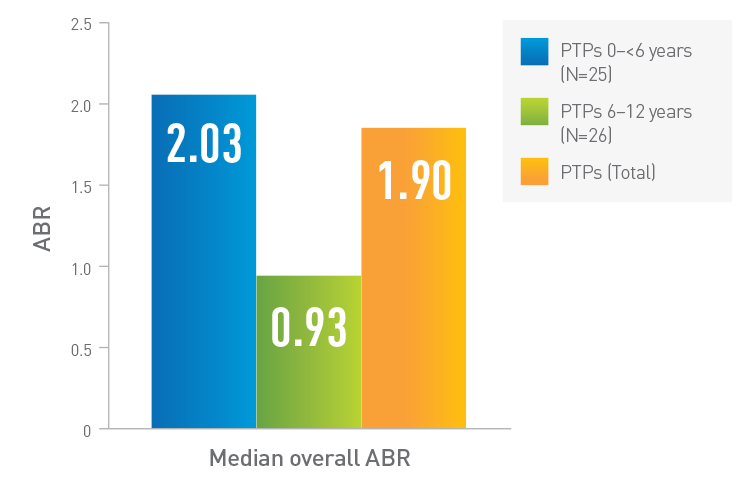
Adapted from Kovaltry Product Monograph and Ljung R, et al.
Dosing frequency
at end of study
(investigator assigned)5
41 %
at 2x weekly
43 %
at 3x weekly
16 %
at every other day
Kovaltry demonstrated efficacy data in ABR for adults
and adolescents1§
ABR in adolescent and adult patients in LEOPOLD I1द
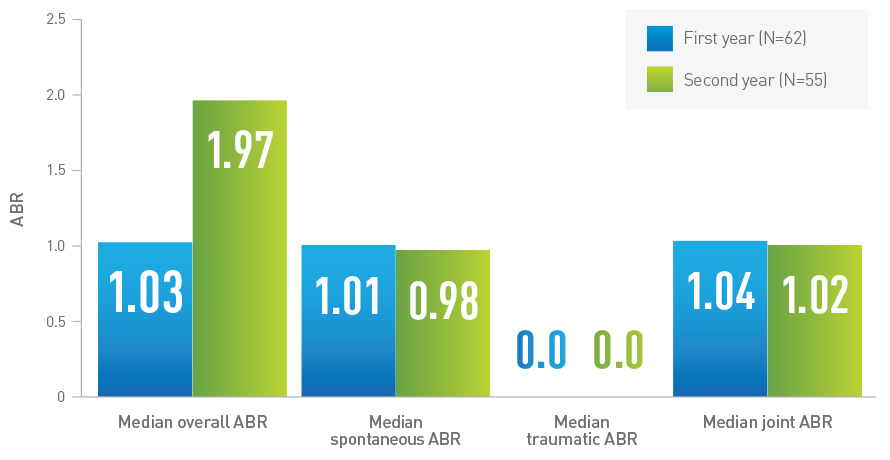
Adapted from Kovaltry Product Monograph.
Dosing frequency
(investigator assigned)1
29 %
at 2x weekly
71 %
at 3x weekly
Kovaltry has demonstrated efficacy data with routine prophylaxis in all of the following age groups:1*§
LEOPOLD Kids
PTPs
0 to <6 years

2.03
Median overall ABR
(Range=0.0, 18.1) (n=25)
PTPs
6 to <12 years

0.93
Median overall ABR
(Range=0.0, 17.7) (n=26)
LEOPOLD I
Adolescents
(>12 years of age) and adults

1.03||
Median overall ABR
(Range=0.0, 26.1) (n=62)
For the LEOPOLD I and LEOPOLD Kids studies, dosing and regimens were determined by physicians.1
Control of bleeding episodes1
Adolescents and adults
- Total bleeding episodes treated with Kovaltry: 1887 in 108 subjects
- Median consumption of Kovaltry for the treatment of breakthrough bleeds in the main studies:
- LEOPOLD I: 28.6 IU/kg/injection (range 13-54 IU/kg)
- LEOPOLD II: 28 IU/kg (range 11-49 IU/kg)
- Majority of bleeding episodes were resolved with one or two infusions of Kovaltry
- 87.6% in LEOPOLD I
- 96.2% and 95.3% in prophylaxis and on-demand in LEOPOLD II
Previously treated children ≤12
- Total bleeding episodes treated with Kovaltry in LEOPOLD Kids Part A main study: 97 in 28 previously treated subjects
- Median consumption of Kovaltry for the treatment of breakthrough bleeds during the 6-month treatment period:
- 36.94 IU/kg/injection (range 20.8–71.6 IU/kg)
- Majority of bleeds were resolved with one to two infusions of Kovaltry (89.7%)
- 92.4% in patients 0 to 6 years of age
- 86.7% in patients 6 to 12 years of age
Previously untreated and minimally treated children ≤12
- Total bleeding episodes treated with Kovaltry in the LEOPOLD Kids Part B main study: 105 of the 184 bleeding episodes in 37 previously untreated and minimally treated subjects
- Majority of bleeds were successfully treated with one to two infusions of Kovaltry (78.1%)
Perioperative management1
- Total major surgeries performed: 11 in 9 previously treated subjects (adults and children)
- Initial Kovaltry doses administered:
- 3000–5000 IU (nominal dose) in the adolescent and adult subjects
- 2500 IU (108.7 IU/kg) was the total initial dose in a single subject ≤12 years of age who underwent a major surgery
- Treatment with Kovaltry provided “good” or “excellent” hemostatic control, as assessed by surgeons
* LEOPOLD Kids (Part A): A two-part, Phase III, multicentre, open-label trial examining the safety, efficacy and pharmacokinetics of Kovaltry in previously treated patients, pediatrics <12 years, with severe hemophilia A (<1%). Patients were given prophylaxis treatment of 25–50 IU/kg at least 2x/week for treatment of breakthrough bleeds and prevention bleeds during surgical procedures.
† Dose/infusion (range) for the control and prevention of bleeding episodes: 38.7 IU/kg (20.8–71.6 IU/kg) for PTPs 0 to <6 years, 32.4 IU/kg (21.7–50.0 IU/kg) for PTPs 6–12 years and 36.9 IU/kg (20.8–71.6 IU/kg) to total PTPs 0–12 years.
‡ Intent-to-treat (ITT) population.
§ LEOPOLD I: A four-part, multicentre, open-label trial examining the safety, efficacy and pharmacokinetics of Kovaltry in previously treated patients, adults and adolescents, with severe hemophilia A (<1%). Patients in Part B were given prophylaxis treatment of 20–50 IU/kg 2–3x/week for 12 months, with two different potency assignments (CS/EP and CS/ADJ for 6 months each).1,3
¶ Median dose/infusion (range) for the control and prevention of bleeding episodes: 31.6 IU/kg (14–67 IU/kg).
|| Data from first year of LEOPOLD I trial.









