Jivi pharmacokinetics1*
- Pharmacokinetics of Jivi were evaluated in previously treated patients (PTPs) (≥18 years of age) with severe hemophilia A following administration of a single 25 IU/kg Jivi and Kogenate® FS dose given twice weekly for 8 weeks1
Jivi demonstrated a longer half-life compared to Kogenate® FS1*
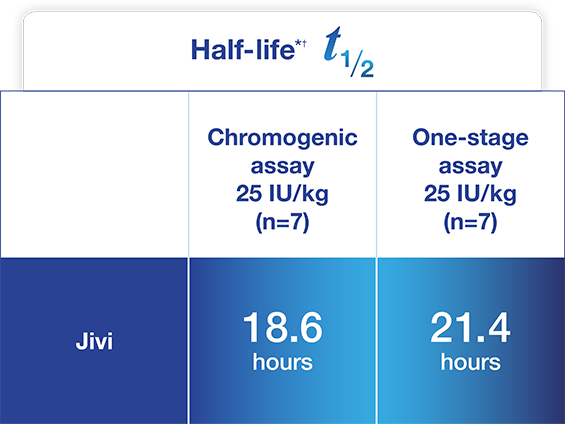
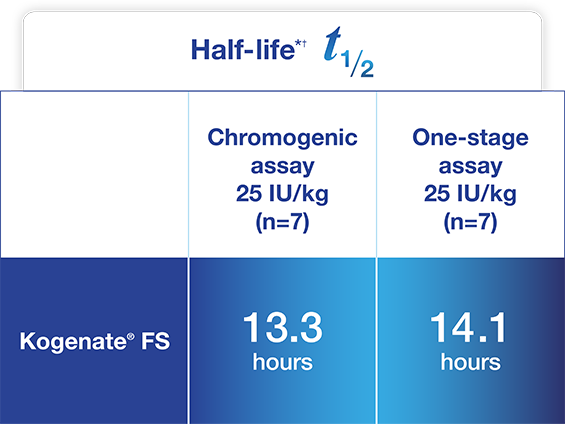
Adapted from Jivi Product Monograph.
Jivi demonstrated reduced clearance and increased AUC compared to Kogenate® FS1,5*
Chromogenic assay (n=7)
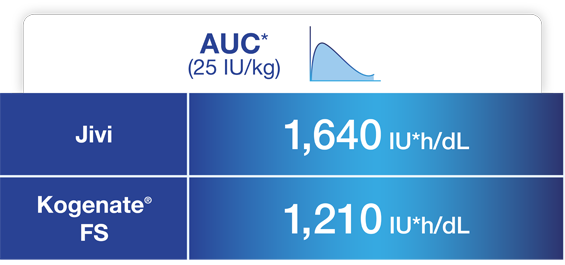
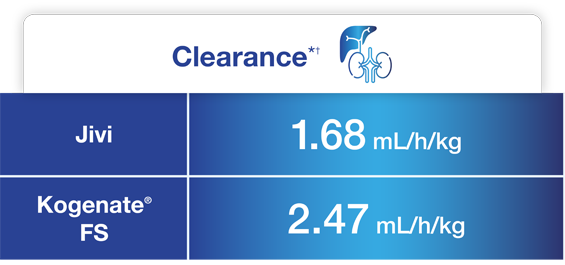
One-stage assay (n=7)
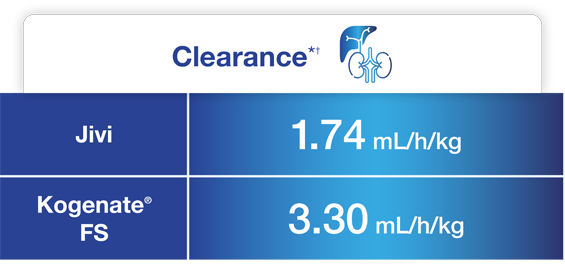
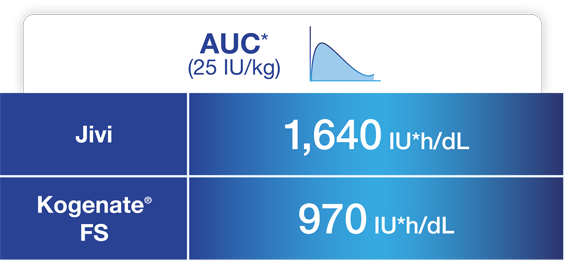
Adapted from Jivi Product Monograph.
~1.4- and 1.5-fold increase in half-life and increased AUC
seen vs. Kogenate® FS based on CS and OS assays, respectively1
* Comparative clinical significance has not been established.
† Arithmetic mean.









