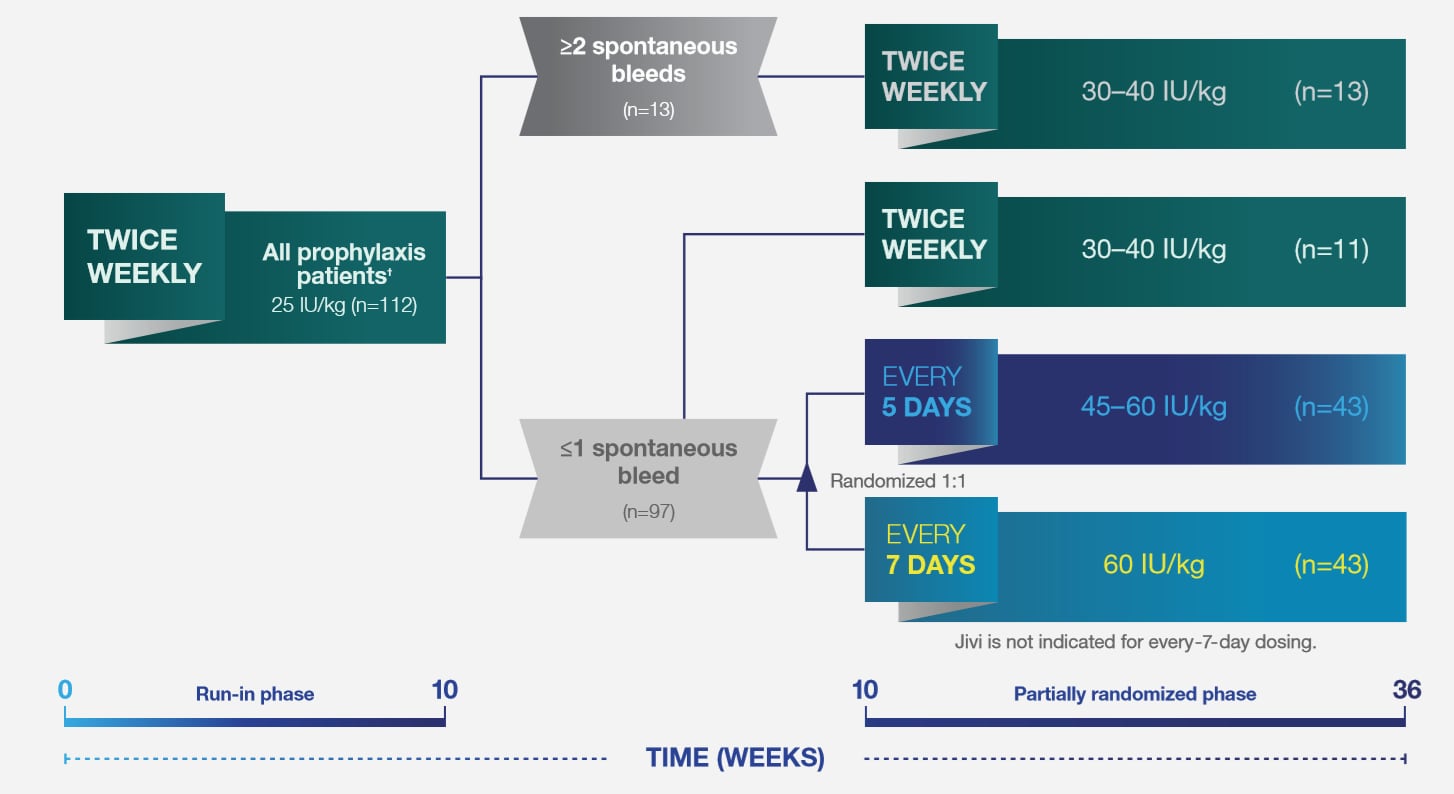
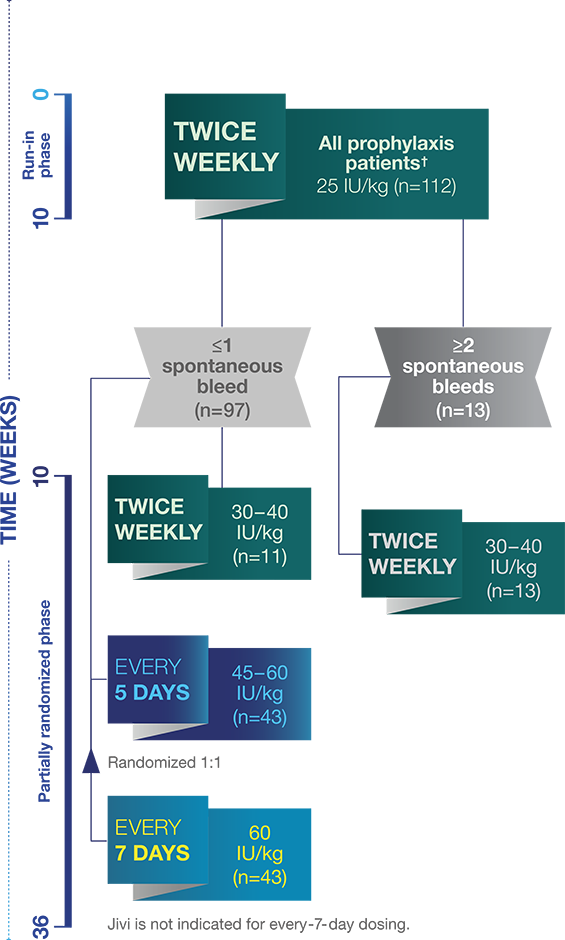
The pivotal PROTECT VIII open-label trial1,6*
Jivi dosing was individualized to patients’ bleeding tendencies1,6
* PROTECT VIII study: A Phase 2/3, multi-national, open-label, uncontrolled, partially randomized study examining the pharmacokinetics, safety and efficacy of Jivi in previously-treated patients (≥150 exposure days [EDs]), adolescents and adults (12 to 65 years of age), with severe hemophilia A (<1%). The main study duration (Part A; n=134) was 36 weeks and evaluated the pharmacokinetics (single dose of 60 IU/kg), safety and efficacy of Jivi for on-demand treatment and routine prophylaxis with three regimens. Safety and efficacy of Jivi in hemostasis during major surgical procedures was evaluated in Part B (n=16). An optional extension study included patients (n=121) completing Part A to accumulate at least 100 EDs. The primary efficacy variable was annualized bleed rate (ABR).
† Comprises all prophylaxis regimens. Two subjects dropped out after single infusion and two additional patients dropped out during the run-in without efficacy data.