Kovaltry pharmacokinetic profile across age groups*
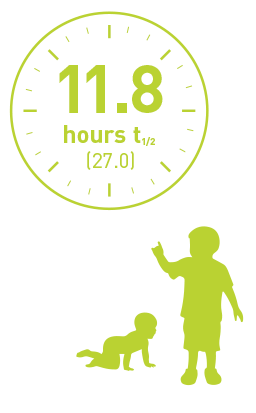
Children (1 to <12 years)1
1 to <6 years
(n=5)
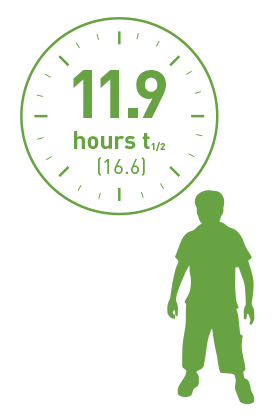
6 to <12 years
(n=10)
All PTPs
(n=15)
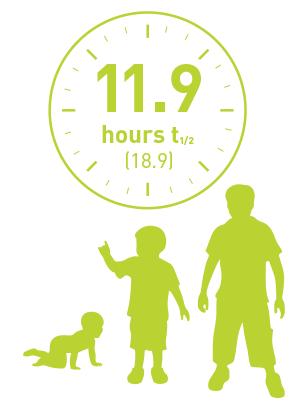
Half-life of pediatrics PTPs (<12 years) using the chromogenic assay.
Geometric mean (%CV).
Adolescents and adults (≥12 years)1
At the beginning
of the study
(n=19)

After 6–12 months
of prophylactic
treatment
(n=19)
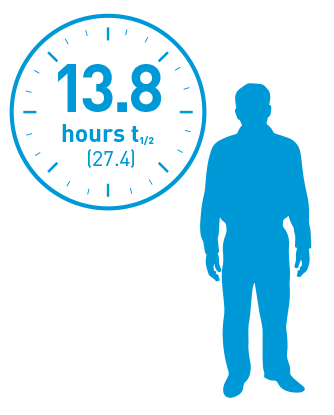
Half-life of adult PTPs (12–62 years) using the one-stage assay.
Geometric mean (%CV).
PK of Kovaltry and selected currently available rFVIII products1,9–11†
Terminal half-life (hours) in children ≤12 years old1,9,10*
11.8
1 to <6 years of age
(n=5)
11.9
6 to <12 years of age
(n=10)
Kovaltry1‡
12.3
<6 years of age
(n=23)
13.5
6 to <12 years of age
(n=31)
Eloctate®9§
11.91
<6 years of age
(n=13)
13.08
6 to <12 years of age
(n=13)
Nuwiq®10¶
Adapted from Kovaltry Product Monograph, Eloctate® Product Monograph and Nuwiq® Product Monograph.
* Clinical significance unknown.
† Data from separate Product Monographs. Comparative clinical significance unknown. Please refer to the respective Product Monographs for complete information.
‡ Half-life of pediatric PTPs (<12 years) using chromogenic assay. Presented in geometric mean.
§ Half-life of pediatric PTPs (<12 years) using one-stage assay. Presented in geometric mean.
¶ Half-life of pediatric PTPs (<12 years) at initial assessment using one-stage assay. Presented in mean.
PTPs=previously treated patients.









