Consider Jivi: a recombinant, B-domain deleted, PEGylated FVIII1

- Efficacy in routine prophylaxis for adults and adolescents with hemophilia A1*
- Proven safety profile: the most-frequently reported adverse reactions in clinical trials were related to headache, cough and pyrexia1
- ~1.4-fold increase in half-life seen compared to Kogenate® FS by CS assay, enabled by site-specifically PEGylated recombinant FVIII that reduces clearance1†

The KoKonnect™ Patient Support Program provides eligible Jivi patients with access to nursing support and/or infusion supplies
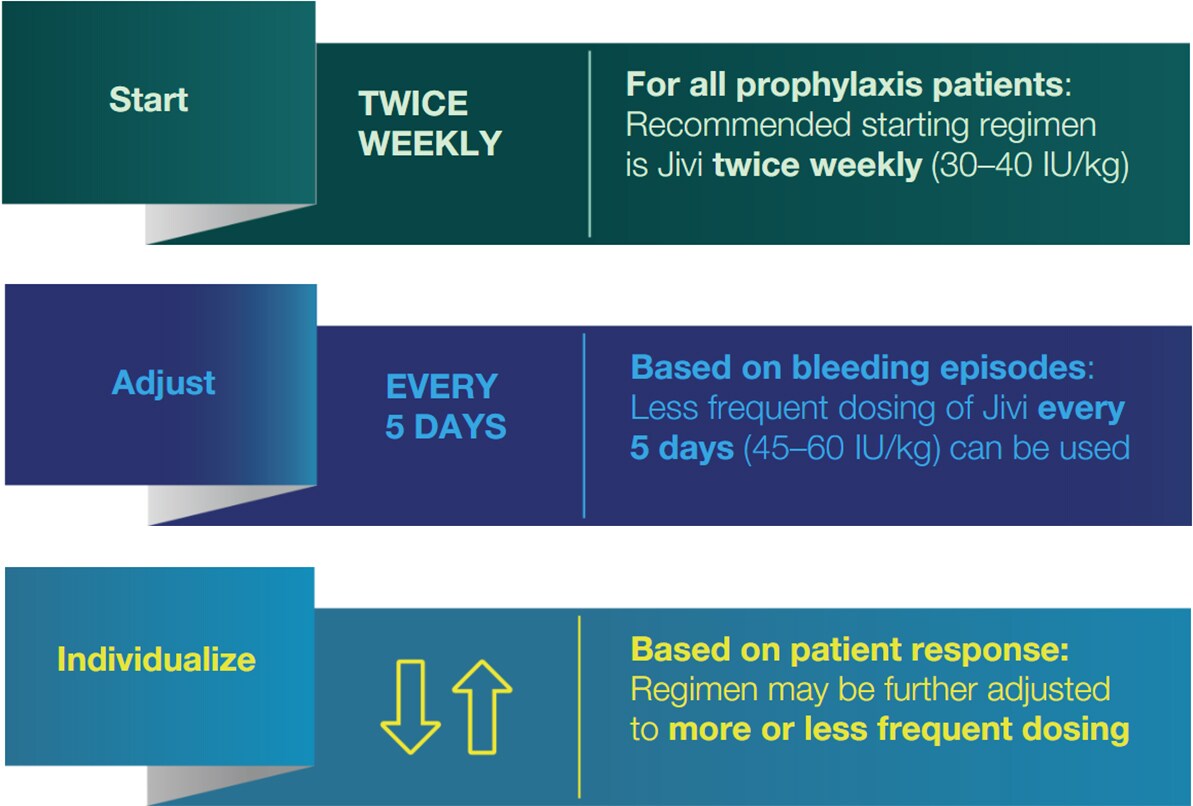
Adjust and individualize dosing based on patient characteristics and treatment response1‡
*PROTECT VIII study: A Phase 2/3, multi-national, open-label, uncontrolled, partially randomized study examining the pharmacokinetics, safety and efficacy of Jivi in previously-treated patients (≥150 exposure days [EDs]), adolescents and adults (12 to 65 years of age), with severe hemophilia A (<1%). The main study duration (Part A; n=134) was 36 weeks and evaluated the pharmacokinetics (single dose of 60 IU/kg), safety and efficacy of Jivi for on-demand treatment and routine prophylaxis with three regimens. Safety and efficacy of Jivi in hemostasis during major surgical procedures was evaluated in Part B (n=16). An optional extension study included patients (n=121) completing Part A to accumulate at least 100 EDs. The primary efficacy variable was annualized bleed rate (ABR).
† Comparative clinical significance has not been established.
‡ Please consult the Product Monograph for full dosing and administration information.










